Presented by: Thanos Sousouras, MD, DO
Edited by: Penelope Burle de Politis, MD

An 88-year-old woman presented with subacutely reduced visual acuity in her left eye.
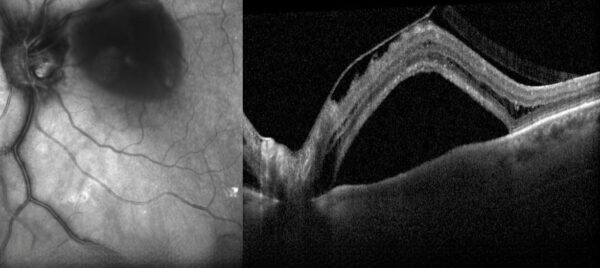
Figure 1: SD-OCT (Heidelberg Engineering®) of the left eye showing the presence of vitreoretinal traction with subretinal and intraretinal fluid.
Case History
An 88-year-old Caucasian woman was referred for investigation of recent-onset central vision loss in her left eye (LE). There were no other accompanying symptoms. Her past ocular history was unremarkable except for uneventful phacoemulsification bilaterally ten years prior. Upon examination, her best-corrected distance visual acuity (BCVA) was 9/10 in the right eye (RE) and 5/10 on the left. Intraocular pressure was within normal limits in both eyes (BE). Biomicroscopy showed no corneal or anterior chamber abnormalities. Color vision was normal, and there was no relative afferent pupillary defect (RAPD). Fundoscopy was suggestive of macular edema on the left. Investigation proceeded with multimodal fundus imaging.
Spectral domain optical coherence tomography (SD-OCT) revealed the presence of vitreoretinal traction with subretinal and intraretinal fluid in the LE (Figure 1).
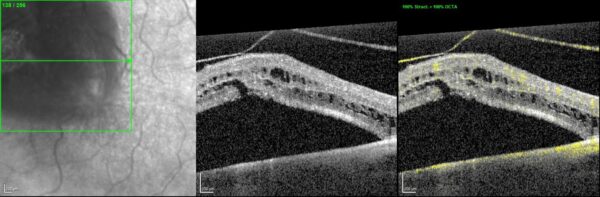
OCT-angiography was inconclusive for the etiological diagnosis (Figure 2).
Figure 2: OCTA macular section scan (Heidelberg Engineering®) of the LE confirming the presence of vitreoretinal traction with subretinal and intraretinal fluid, without any other relevant signs.
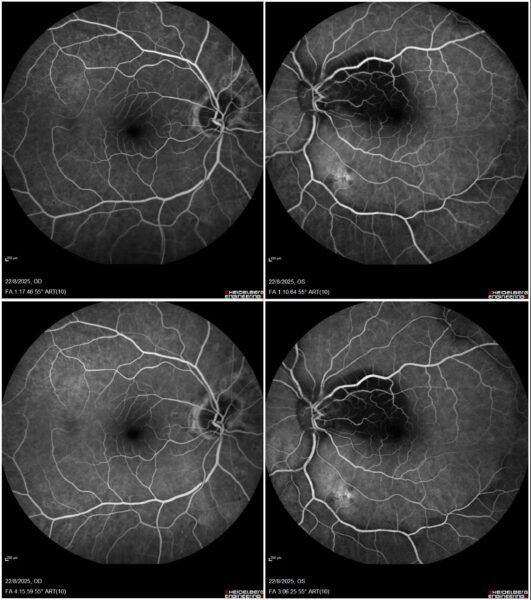
Fundus fluorescein angiography (FFA) showed extensive macular hypofluorescence and a single atrophic lesion in the left eye – between the inferior vascular arcades – without significant change in fluorescence through the angiographic phases (Figure 3).
Figure 3: Bilateral 55° field FFA (Heidelberg Engineering®) featuring extensive macular hypofluorescence and a single, non-exudative posterior pole lesion in the left eye, without significant change in fluorescence through the early- (top) and late-phase (bottom) angiograms.
Additional History
Based on the clinical presentation and multimodal image findings of macular edema with vitreoretinal traction, the surgical approach through pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling was indicated.
Differential Diagnosis of Vitreoretinal Traction-Induced Macular Edema
- diabetic macular edema
- age-related macular degeneration (AMD)
- epiretinal membrane (ERM)
- retinal vein occlusions (RVOs)
- uveitis
- central serous chorioretinopathy (CSR)
- retinoschisis
- macular telangiectasia
- idiopathic juxtafoveal telangiectasia
- retinal tumors
- medication-induced macular edema
Clinical history and examination, aided by multimodal fundus imaging, are the key to early, accurate diagnosis and better follow-up and interpretation of macular edema and vitreoretinal interface disorders.
Discussion and Literature
Macular edema (ME) is a retinal condition characterized by fluid buildup in the central part of the retina, causing decreased visual acuity and, when persistent, leading to severe vision loss. Fluid accumulates due to a mismatch between retinal fluid entry and exit mechanisms. The underlying pathogenesis is a dysfunction of the blood-retinal barrier, allowing the entry of proteins and solutes into the retinal tissue.
The distinctive anatomical characteristics of the macula, including its abundant photoreceptor count, elevated metabolic activity, and limited extracellular fluid resorption due to a central avascular zone, along with its specialized cellular and molecular composition containing specific glial cells like Müller cells, suggest a propensity for fluid accumulation. Furthermore, the intriguing arrangement of the Henle fiber layer and the potential presence of a paravascular “glymphatic” waste clearance pathway also indicate the macula’s role as a reservoir for fluid retention.
Macular edema can result from different intraocular and systemic insults, most commonly following intraocular surgery, venous occlusive disease, diabetic retinopathy, and posterior segment inflammatory disease. The following parameters are relevant for clinical evaluation of ME: extent, distribution (focal versus diffuse); central foveal involvement (500 μm-area), fluorescein leakage and intraretinal cysts, signs of ischemia, presence or absence of vitreous traction, increase in retinal thickness, and chronicity.
Symptoms of ME include metamorphopsia, micropsia, blurred vision, a central scotoma, and reduction of contrast or color sensitivity. The clinical diagnosis of ME can be challenging in mild cases or when visualization of the fundus is impaired by poor pupillary dilation, cataract and other ocular media opacities. Therefore, multimodal imaging methods are commonly employed. Fluorescein angiography and optical coherence tomography provide enhanced visualization of the geometry and distribution of ME.
Therapeutic approaches depend on the underlying pathology and include topical and systemic steroids and non-steroidal anti-inflammatory agents, laser photocoagulation, immunomodulators, intravitreal injection of triamcinolone, dexamethasone implant, and pars plana vitrectomy. Pars plana vitrectomy is mostly reserved for treating mechanical factors contributing to ME, such as epiretinal membranes and vitreoretinal traction.
Traction ME may develop through contraction of macular epiretinal membranes (ERM), or due to persistent vitreomacular traction during the evolution of vitreomacular traction syndrome (VMS). Vitreomacular traction (VMT) is characterised by partial anomalous posterior vitreous detachment that causes anteroposterior traction on the macula in areas of residual vitreous adhesion. The adherent vitreous cortex results in a broad, often dumbbell-shaped region encompassing the macular area and optic nerve. This in turn causes cystoid thickening of the macula. These patients are best managed surgically rather than medically.
The beneficial effect of vitrectomy with release of traction on the macula is explained by an increase in tissue pressure and a lowering of the hydrostatic pressure gradient, reducing the water flux from blood vessels into retinal tissue. The therapeutic action of vitrectomy in nontractional edema is thought to be based on two mechanisms: increased oxygen transport between the anterior and posterior segments of the eye and the removal of growth factors which are secreted in large amounts into the vitreous during proliferative vasculopathies.
Several innovations propelled vitreoretinal surgery forward over the past several years, including endoscopic light amplification by stimulated emission of radiation (endoLASER), perfluorocarbon liquid, widefield viewing systems, and improved illuminating systems (e.g., Xenon). Each of these advances made it easier to treat diseases that were previously untreatable. PPV with ILM peeling has been proved to be an effective treatment for ME across various etiologies.
Keep in mind
- Macular edema is a nonspecific sign of ocular disease and not a specific entity. It should be viewed as a macular response to an altered retinal environment, usually an alteration of the blood-retinal barrier.
- Early diagnosis and proper management of macular edema, according to its etiology, are essential to avoid chronicity and preserve visual function.
- Vitreoretinal surgical techniques can be effective in the management of macular edema associated with pathology of the vitreous body.
References
- Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I & Pavesio CE (2004). Macular edema. Survey of ophthalmology, 49(5), 470–490. https://doi.org/10.1016/j.survophthal.2004.06.002
- Kohli, P., Tripathy, K., & Patel, B. C. (2024). Macular Edema. In StatPearls. StatPearls Publishing.
- Haydinger CD, Ferreira LB, Williams KA & Smith JR (2023). Mechanisms of macular edema. Frontiers in medicine, 10, 1128811. https://doi.org/10.3389/fmed.2023.1128811
- Coscas G, Cunha-Vaz J & Soubrane G (2017). Macular Edema: Definition and Basic Concepts. Developments in ophthalmology, 58, 1–10. https://doi.org/10.1159/000455264
- Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild PR, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P & Behar-Cohen F (2018). Mechanisms of macular edema: Beyond the surface. Progress in retinal and eye research, 63, 20–68. https://doi.org/10.1016/j.preteyeres.2017.10.006
- Trichonas G & Kaiser PK (2014). Optical coherence tomography imaging of macular oedema. The British journal of ophthalmology, 98 Suppl 2(Suppl 2), ii24–ii29. https://doi.org/10.1136/bjophthalmol-2014-305305
- Wolfensberger TJ (2017). Macular Edema – Rationale for Therapy. Developments in ophthalmology, 58, 74–86. https://doi.org/10.1159/000455275
- Pournaras CJ, Kapetanios AD & Donati G (1999). Vitrectomy for traction macular edema. Documenta ophthalmologica. Advances in ophthalmology, 97(3-4), 439–447. https://doi.org/10.1023/a:1002408206561
- Golan S & Loewenstein A (2014). Surgical treatment for macular edema. Seminars in ophthalmology, 29(4), 242–256. https://doi.org/10.3109/08820538.2013.796394
- El-Khoury S, Abdelmassih Y, Ducournau D, Drimbea A, Portmann A & SCRV macular edema study group (2025). Pars plana vitrectomy with ILM peeling for macular edema of various etiologies: results from the SCRV macula surgery study. International ophthalmology, 45(1), 352. https://doi.org/10.1007/s10792-025-03721-0