Presented by: Paris Tranos MD, PhD, ICOphth, FRCS

Edited by: Penelope Burle de Politis, MD

A 34-year-old woman presented with long-standing flashes and floaters in the right eye and recent-onset similar symptoms in the left eye.
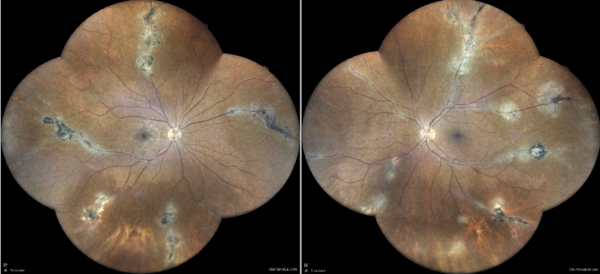
Figure 1: Widefield color fundus photograph (EIDON true-color confocal scanner®) displaying grayish perivascular lesions in combination with depigmented and atrophic retinal pigment epithelium.
Case History
A 34-year-old Caucasian woman presented with a one-year history of flashes and floaters in the right eye (RE) and recent onset (3 weeks) of similar symptoms in the left eye (LE). Her past medical history included gestational diabetes, autoimmune thyroiditis, femoropopliteal bypass graft due to a road traffic accident, skin lesions with spontaneous regression one year prior, and recurrent oral ulcers biannually. She was on oral anticoagulant medication. Her family history was notable for rheumatoid arthritis.
Upon examination, her corrected distance visual acuity (CDVA) was 10/10 in both eyes (BE). Intraocular pressure was within the normal range bilaterally, and anterior segment biomicroscopy was unremarkable. Color vision was normal, and there was no relative afferent pupillary defect (RAPD). Mild vitritis was noted bilaterally, more prominent in the RE.
Investigation proceeded with multimodal fundus imaging. Widefield retinography revealed multiple atrophic lesions along peripheral venous branches in both eyes (Figure 1). Fundus autofluorescence (FAF) detected hypoautofluorescence in atrophic areas and hyperautofluorescence alongside the affected vascular segments (Figures 2 and 3).
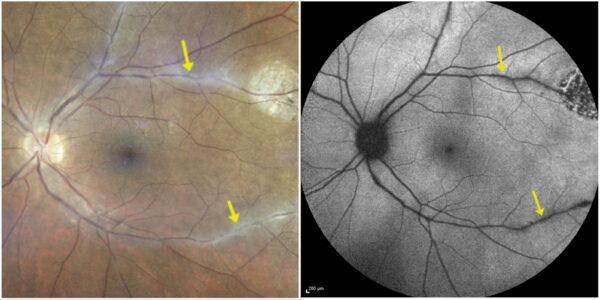
Figure 2: Fundus photograph (EIDON true-color confocal scanner®, left) and fundus autofluorescence (Heidelberg Engineering®, right) of the left eye illustrating the correspondence of the perivascular lesions in multimodal imaging.
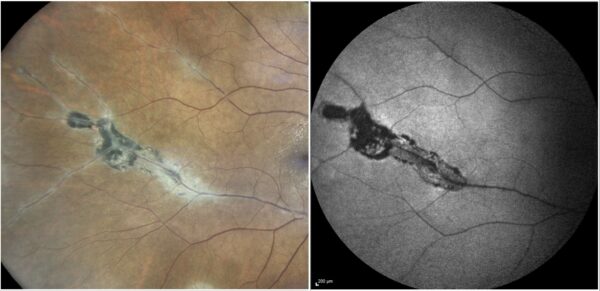
Figure 3: High magnification of fundus photograph (left) and fundus autofluorescence (right) depicting detailed aspects of the perivascular chorioretinal atrophic lesions.
Fundus fluorescein angiography (FFA) demonstrated bilateral optic disc fluorescein leakage, diffuse and segmental retinal phlebitis, and focal dye leak at the atrophic perivascular areas (Figure 4).
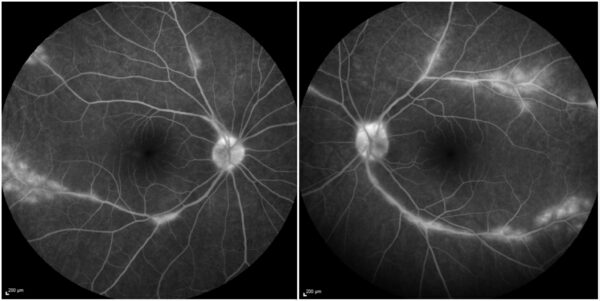
Figure 4: Fundus fluorescein angiography (Heidelberg Engineering®) demonstrating fluorescein leakage at the optic disc, diffuse and segmental vasculitis involving the retinal veins, and focal fluorescein leakage at the perivascular lesions in both eyes.
Based on medical history, clinical examination, and multimodal image findings, the diagnosis of pigmented paravenous retinochoroidal atrophy (PPRCA) was established. Systemic investigation, including HLA-B51 testing and chest radiography, showed no abnormalities, except for positive blood antinuclear antibodies (ANA).
Treatment with oral steroids (prednisolone) was initiated, and the patient was referred for rheumatologic evaluation. At one-month follow-up, the patient was asymptomatic, with regression of disease activity as observed on fundus examination and fluorescein angiography (Figure 5).
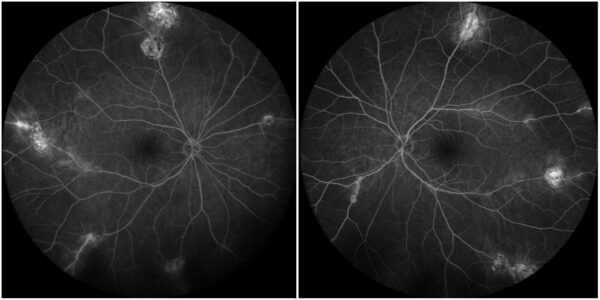
Figure 5: Fundus fluorescein angiography showing absence of active leakage from the optic disc, large veins, and atrophic perivascular lesions in both eyes
Additional History
Long-term management involved immunosuppressive therapy with adalimumab, which was subsequently changed to mycophenolic acid and azathioprine due to the development of anti-drug antibodies. Although disease exacerbations were controlled, and preservation of visual acuity (UCVA 10/10 in BE) was achieved, progression of the fundus lesions was noted over the course of 5.5 years (Figure 6).
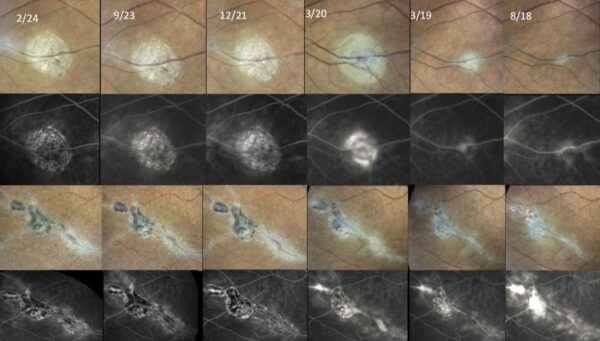
Figure 6: Serial fundus photographs (first and third rows) and fluorescein angiograms (second and fourth rows) of the same two perivenous retinochoroidal lesions, along 5.5 years of follow-up (from right to left). Notice the progression of atrophic changes despite the symptomatic improvement provided by therapy.
Differential Diagnosis of Pigmented Paravenous Retinochoroidal Atrophy
- sarcoidosis
- tuberculosis
- syphilis
- acute zonal occult outer retinopathy (AZOOR)
- autoimmune retinopathies (AIR)
- CRB1 (Crumbs homolog 1) gene-associated retinal degeneration
- acute behavioral disturbance (ABD)
The differential diagnosis of PPRCA with other chorioretinopathies is based on careful history taking and clinical examination. The lack of other affected family members, suggesting an acquired rather than inherited disorder, along with a positive response to systemic immunosuppression, favors the diagnosis of PPRCA.
Discussion and Literature
Pigmented Paravenous Retinochoroidal Atrophy (PPRCA) is a rare, chronic, and often asymmetric chorioretinopathy characterized by pigment clumping and retinal pigment epithelium (RPE) atrophy distributed along the retinal veins, predominantly in the periphery. First described by Brown in 1937 as “retinochoroiditis radiata,” the condition is typically discovered incidentally during routine examination, as many patients remain asymptomatic. When symptoms do occur, they include photopsia, blurred vision, peripheral visual field loss, and nyctalopia. Central visual acuity is generally preserved unless the macula is involved.
While the etiology remains unclear, PPRCA is typically sporadic and shows no gender predilection. Genetic, infectious, and inflammatory mechanisms have been proposed. Cases associated with conditions like uveitis, Behçet’s disease, congenital syphilis, and viral illnesses (including COVID-19) are now regarded as pseudo-PPRCA, where the retinal changes are secondary to inflammation.
Multimodal imaging is essential for diagnosis and monitoring. Fundus autofluorescence (FAF) typically reveals paravenous hypoautofluorescence with surrounding hyperautofluorescence. Fundus fluorescein angiography (FFA) may show capillary non-perfusion and fluorescein leakage, the latter indicating active inflammation. Optical coherence tomography (OCT) often reveals thinning of the outer retina, RPE, and choroid. OCT-angiography (OCT-A) has demonstrated deep capillary plexus disruption, supporting the theory that ischemia may precede photoreceptor and RPE degeneration. Changes in the choriocapillaris may reflect secondary involvement.
Electroretinography (ERG) often shows bilateral rod and cone dysfunction, with notable interocular asymmetry. Visual field testing typically reveals peripheral defects corresponding to areas of retinal atrophy. Ancillary lab testing is important to exclude mimicking inflammatory or infectious conditions.
PPRCA has been categorized into three patterns: paravenous, with pigment clumping along veins; focal, with isolated lesions; and confluent, resembling retinitis pigmentosa due to extensive pigment deposition.
Despite treatment, the condition is slowly progressive. Immunosuppressive therapy may reduce disease activity—as suggested by fluorescein leakage—but does not halt the progression of atrophic changes. Thus, the primary therapeutic goal is symptom control and preservation of visual function.
Keep in mind
- PPRCA is a rare form of chronic, usually asymmetric chorioretinopathy occurring mostly around peripheral retinal veins and rarely affecting visual acuity.
- PPRCA is best approached with multimodal retinal imaging. Fluorescein leakage in FFA is indicative of disease activity.
- Regardless of symptomatic relief, the atrophic lesions in PPRCA progress despite immunosuppressive therapy.
References
- Antropoli A, Arrigo A, Pili L, Bianco L, Berni A, Saladino A, Bandello F & Battaglia Parodi M (2024). Pigmented paravenous chorioretinal atrophy: Updated scenario. European journal of ophthalmology, 34(4), 941–951. https://doi.org/10.1177/11206721231199118
- Ranjan R, Jain MA, Verghese S, Manayath GJ & Narendran V (2022). Multimodal imaging of pigmented paravenous retinochoroidal atrophy. European journal of ophthalmology, 32(1), NP125–NP129. https://doi.org/10.1177/1120672120965489
- Battaglia Parodi M, Arrigo A, Chowers I, Jarc-Vidmar M, Shpigel M, Bandello F & Michaelidis M (2022). OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY FINDINGS IN PIGMENTED PARAVENOUS CHORIORETINAL ATROPHY. Retina (Philadelphia, Pa.), 42(5), 915–922. https://doi.org/10.1097/IAE.0000000000003407
- Ramtohul P, Chehaibou I & Bonnin S (2022). PERIPHERAL RETINAL VASCULAR ABNORMALITIES IN PIGMENTED PARAVENOUS RETINOCHOROIDAL ATROPHY. American journal of ophthalmology, 236, e4–e5. https://doi.org/10.1016/j.ajo.2022.01.003
- Lee EK, Lee SY, Oh BL, Yoon CK, Park UC & Yu HG (2021). Pigmented Paravenous Chorioretinal Atrophy: Clinical Spectrum and Multimodal Imaging Characteristics. American journal of ophthalmology, 224, 120–132. https://doi.org/10.1016/j.ajo.2020.12.010
- Gasparian S & Sierpina DI (2021). Fundus autofluorescence detects subtle pigmentary alterations in pigmented paravenous retinochoroidal atrophy. American journal of ophthalmology case reports, 23, 101158. https://doi.org/10.1016/j.ajoc.2021.101158
- Shona OA, Islam F, Robson AG, Webster AR, Moore AT & Michaelides M (2019). PIGMENTED PARAVENOUS CHORIORETINAL ATROPHY: Detailed Clinical Study of a Large Cohort. Retina (Philadelphia, Pa.), 39(3), 514–529. https://doi.org/10.1097/IAE.0000000000001950
- Sato T, Takenaka Y & Takeuchi M (2024). Bilateral Perivascular Chorioretinal Atrophy Resembling Pigmented Paravenous Chorioretinal Atrophy Post COVID-19 Infection: A Case Report and Comprehensive Immune Profiling. Vaccines, 12(8), 878. https://doi.org/10.3390/vaccines12080878
- Barteselli G (2014). Fundus autofluorescence and optical coherence tomography findings in pigmented paravenous retinochoroidal atrophy. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie, 49(6), e144–e146. https://doi.org/10.1016/j.jcjo.2014.08.019
- Choi JY, Sandberg MA & Berson EL (2006). Natural course of ocular function in pigmented paravenous retinochoroidal atrophy. American journal of ophthalmology, 141(4), 763–765. https://doi.org/10.1016/j.ajo.2005.11.009


