Presented by: Miltos Balidis MD, PhD, FEBOphth, ICOphth
Edited by: Penelope de Politis, MD
A 31-year-old woman presented with corneal scarring after a SARS-CoV-2 infection.
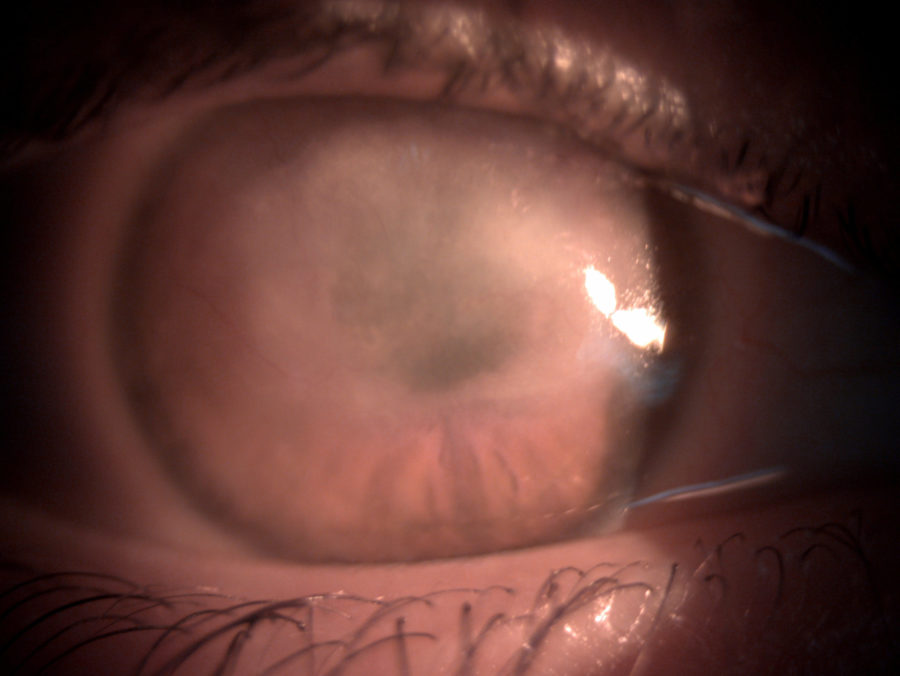
Figure 1: Wide beam slit lamp photography of the right eye displaying diffuse scarring of the corneal upper two thirds.
Case History
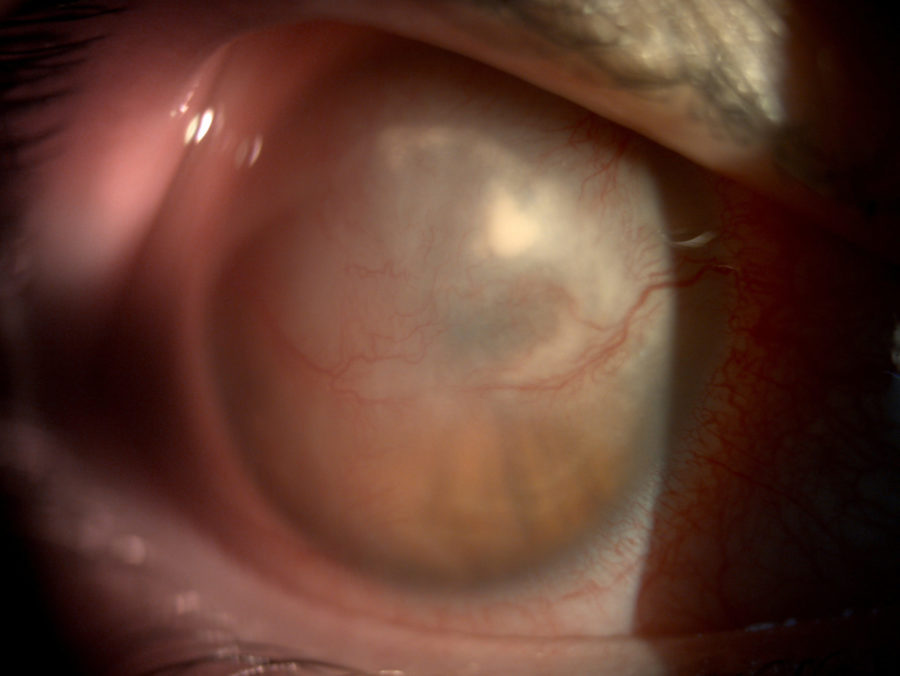
A 31-year-old Caucasian woman presented with extensive corneal scarring in the right eye (RE) after a 2-month permanence at an intensive care unit (ICU) for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The patient had been treated with an eye drop combination of gentamicin and dexamethasone. She had no previous history of ocular diseases or visual deficiency. Her family history was unremarkable. Ophthalmological examination revealed a corrected distance visual acuity (CDVA) of finger count (FC) in the RE and 10/10 in the left eye (LE). Under biomicroscopy, the RE featured extensive stromal fibrosis involving the upper ⅔ of the cornea, measuring 10 x 4 mm in the largest axes (Figure 1), compromising the pupillary area, with residual stromal infiltrates and neovascularization at 180° of the corneal circumference (Figure 2). Intraocular pressure was 17 mmHg in the RE and 15 mmHg in the LE. Fundoscopy was unfeasible in the RE but normal in the LE.
Figure 2: Slit lamp photography with a higher magnification showing sparse stromal infiltrates at the mid-periphery of the cornea and prominent neovascularization over the area of corneal scarring.
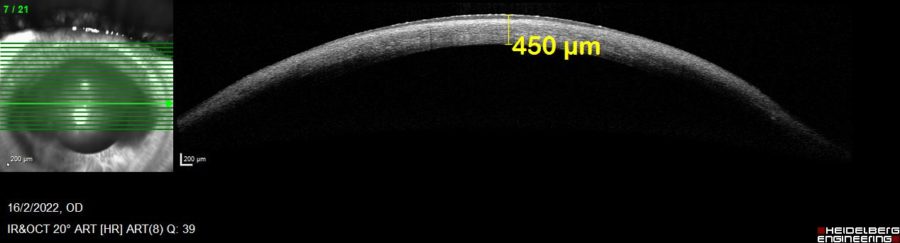
Anterior segment spectral domain optical coherence tomography (anterior SD-OCT) showed diffuse reduction of corneal pachymetry (Figure 3), indicating that the scar had developed over a ground of previous tissue loss, probably corneal melting.
Figure 3: Anterior SD-OCT (Heidelberg Engineering®) of the RE showing the change in corneal architecture with extensive thinning (central pachymetry of 450 μm).
The patient was put on dexamethasone drops for the RE every 4 hours and scheduled for review in 8 weeks. Upon return, UCVA had improved to 0.3/10. Corneal pachymetry remained stable, and the infiltrates were no longer noticeable; therefore, a slow tapering of the corticotherapy was started.
However, on a second follow-up visit 3 weeks later, the patient presented with a hyperemic RE of a week duration, and the biomicroscopic examination revealed new corneal infiltrates (Figure 4).
Figure 4: Slit lamp photography of the RE showing a broad stromal infiltrate involving the whole superior nasal quadrant of the cornea, with diffuse conjunctival injection and exacerbation of the neovessels in the area of corneal scarring.
Additional History
The patient had a history of labial herpes. Even though the lesion was not typical of an intermediate herpetic keratitis, the absence of other risk factors such as trauma or contact lens wear, added to the corneal change with diffuse thinning, scarring and neovessels, led to the suspicion of a herpetic etiology. For this reason, the dexamethasone drops were restarted and valacyclovir p.o. (1g b.i.d.) was added to the drug regimen.
A week later, the infiltrates had subsided, vision was stable and there was no evidence of further corneal thinning on the OCT. Due to the temporal correlation with the ICU admission for SARS-CoV-2 treatment and considering the numerous reports on increased susceptibility and reactivation of latent infection by the entire herpesviridae family in COVID-19 patients, the diagnosis of severe herpetic keratitis induced by SARS-CoV-2 was established.
Medication was sustained according to the current protocols for intermediate herpetic keratitis, and the patient will be checked-up closely for the regression of the acute corneal picture, so that a plan can be drawn in the future in order to improve her visual acuity in the affected eye.
Differential Diagnosis of Corneal Scarring
- trauma
- chemical injuries
- infections (viral, fungal, bacterial, protozoal)
- surgeries
- dystrophies
- keratoconus
- ocular surface disorders (e.g., severe dry eye)
- ocular cicatricial pemphigoid
The differentiation among the possible causes of corneal scarring is crucial in determining an accurate diagnosis and proper management. Knowledge of the pathophysiological basis of tissue scarring, a thorough history (present and past, ocular and systemic), and careful corneal examination including high-resolution methods may be extremely useful in guiding the ophthalmologist towards the right etiology.
Discussion and Literature
Injuries to the cornea produce varying amounts of corneal scarring depending on the magnitude of cellular and molecular responses to injury. The pathophysiological process that leads to corneal scarring can be attributable to corneal fibroblast and/or myofibroblast generation, with or without neovascularization (as triggered by hypoxia, inflammation, and/or infection), typically following trauma, chemical burns, infections, surgeries, and corneal or ocular surface disorders.
One of the most prevalent infectious causes of corneal scarring is herpes simplex. The virus can manifest at any of the corneal layers, leading to more or less severe complications depending on the depth and extension of corneal involvement. Stromal herpetic keratitis is a leading cause of corneal scarring, with the consequent loss of corneal transparency and visual acuity.
COVID-19 and anti-COVID-19 vaccines have been proved to affect the eye in several different ways, ranging from mild conjunctivitis to corneal graft rejection. Among COVID-19 patients, the prevalence of ophthalmic manifestations ranges from 2-32%. The mechanism involved in each ocular manifestation can be of inflammatory and/or immunological basis. It has been shown that corneal and conjunctival cells show co-expression of the TMPRSS2 and ACE2 genes involved in COVID-19 infection, though at a lower concentration than in other tissues such as the nasal cavity or lung parenchyma. Acute-onset bilateral interstitial keratitis induced by COVID-19 has been reported.
Recent findings have pointed to a clear association between COVID-19 and an increased incidence of cases involving the entire herpesviridae family (i.e., Epstein-Barr virus, cytomegalovirus, varicella-zoster virus, herpes simplex virus, human-herpes viruses, Kaposi’s sarcoma virus), especially in critically ill patients. It is possible that the use of corticosteroids in the management of COVID-19 infection facilitates the emergence of herpes as an opportunistic manifestation. Moreover, it has been demonstrated that some of the new antiviral drugs used to treat SARS-CoV-2 infection can induce reactivation of some of the herpesviridae viruses. Reactivation of herpes viruses has also been reported following anti-COVID-19 vaccination.
Though presently limited to a few case reports, the induction of corneal herpes is a potential COVID-19-associated condition, which may present either during the acute or in the convalescent phase of the illness. In mild cases, COVID-19-induced herpetic keratitis can be restricted to the epithelium and subside leaving no scarring or visual loss. However, in patients staying in ICUs due to SARS-CoV-2, the possibility of deeper corneal involvement must always be considered in order to ensure prompt management, thus avoiding or minimizing vision-threatening corneal opacification. Differential diagnosis with other infectious etiologies facilitated by SARS-CoV-2 must be made, notably fungus and atypical opportunistic microorganisms. Polymerase chain reaction (PCR) tests and even biopsy may be necessary in doubtful or refractory cases.
Keep in mind
- Herpetic keratitis is one the most common causes of corneal scarring.
- COVID-19 increases susceptibility to infection and reactivation of latent viruses of the entire herpesviridae family, including herpes simplex in the eye.
- COVID-19 and its related vaccines must now be considered among the possible etiologies of acute ocular manifestations of obscure causes.
References
- Wilson SE, Sampaio LP, Shiju TM, Hilgert G & de Oliveira RC (2022). Corneal Opacity: Cell Biological Determinants of the Transition From Transparency to Transient Haze to Scarring Fibrosis, and Resolution, After Injury. Investigative ophthalmology & visual science, 63(1), 22. https://doi.org/10.1167/iovs.63.1.22
- Sen M, Honavar SG, Sharma N & Sachdev MS (2021). COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian journal of ophthalmology, 69(3), 488–509. https://doi.org/10.4103/ijo.IJO_297_21
- Balidis M, Mikropoulos D, Gatzioufas Z, de Politis PB, Sidiropoulos G & Vassiliadis V (2021). Acute corneal graft rejection after anti-severe acute respiratory syndrome-coronavirus-2 vaccination: A report of four cases. European journal of ophthalmology, 11206721211064033. Advance online publication. https://doi.org/10.1177/11206721211064033
- Schnichels S, Rohrbach JM, Bayyoud T, Thaler S, Ziemssen F & Hurs J (2021). Can SARS-CoV-2 infect the eye? An overview of the receptor status in ocular tissue. Kann SARS-CoV-2 das Auge infizieren? – Ein Überblick über den Rezeptorstatus in okularem Gewebe. English version. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft, 118(Suppl 1), 81–84. https://doi.org/10.1007/s00347-020-01281-5
- Cano-Ortiz A, Leiva-Gea I, Ventosa ÁS, González-Cruces T, Sánchez-González JM, Morales P & Villarrubia A (2022). Stromal interstitial keratitis in a patient with COVID-19. Journal français d’ophtalmologie, 45(4), e175–e177. https://doi.org/10.1016/j.jfo.2021.11.004
- Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadié JM & Reizine F (2020). Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Critical care (London, England), 24(1), 530. https://doi.org/10.1186/s13054-020-03252-3
- Honore PM, Barreto Gutierrez L, Kugener L, Redant S, Attou R, Gallerani A & De Bels D (2020). SARS-CoV-2 infection as a risk factor for herpesviridae reactivation: consider the potential influence of corticosteroid therapy. Critical care (London, England), 24(1), 623. https://doi.org/10.1186/s13054-020-03349-9
- Simonnet A, Engelmann I, Moreau AS, Garcia B, Six S, El Kalioubie A, Robriquet L, Hober D & Jourdain M (2021). High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infectious diseases now, 51(3), 296–299. https://doi.org/10.1016/j.idnow.2021.01.005
- Im JH, Nahm CH, Je YS, Lee JS, Baek JH, Kwon HY, Chung MH, Jang JH, Kim JS, Lim JH & Park MH (2022). The effect of Epstein-Barr virus viremia on the progression to severe COVID-19. Medicine, 101(18), e29027. https://doi.org/10.1097/MD.0000000000029027
- Chen J, Dai L, Barrett L, James J, Plaisance-Bonstaff K, Post SR & Qin Z (2021). SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Communications biology, 4(1), 682. https://doi.org/10.1038/s42003-021-02220-z
- Fathy R A, McMahon D E, Lee C, Chamberlin GC, Rosenbach M, Lipoff JB, Tyagi A, Desai SR, French LE, Lim HW, Thiers BH, Hruza GJ, Fassett M, Fox LP, Greenberg HL, Blumenthal K & Freeman EE (2022). Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. Journal of the European Academy of Dermatology and Venereology : JEADV, 36(1), e6–e9. https://doi.org/10.1111/jdv.17646
- Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J & Kolar P (2021). Herpes Simplex Keratitis in Patients with SARS-CoV-2 Infection: A Series of Five Cases. Medicina (Kaunas, Lithuania), 57(5), 412. https://doi.org/10.3390/medicina57050412
- Das N, Das J & Pal D (2022). Stromal and endothelial herpes simplex virus keratitis reactivation in the convalescent period of COVID-19 – A case report. Indian journal of ophthalmology, 70(4), 1410–1412. https://doi.org/10.4103/ijo.IJO_2838_21
- You IC, Ahn M & Cho NC (2022). A Case Report of Herpes Zoster Ophthalmicus and Meningitis After COVID-19 Vaccination. Journal of Korean medical science, 37(20), e165. https://doi.org/10.3346/jkms.2022.37.e165
- Roy A, Chaurasia S, Ramappa M, Joseph J & Mishra DK (2022). Clinical profile of keratitis treated within 3 months of acute COVID-19 illness at a tertiary care eye center. International ophthalmology, 1–9. Advance online publication. https://doi.org/10.1007/s10792-022-02288-4