Presented by: Evangelos Lokovitis MD, FEBOphth

Edited by: Penelope Burle de Politis, MD
A 66-year-old man presented with rapid-onset exophthalmos and lacrimation on the left.
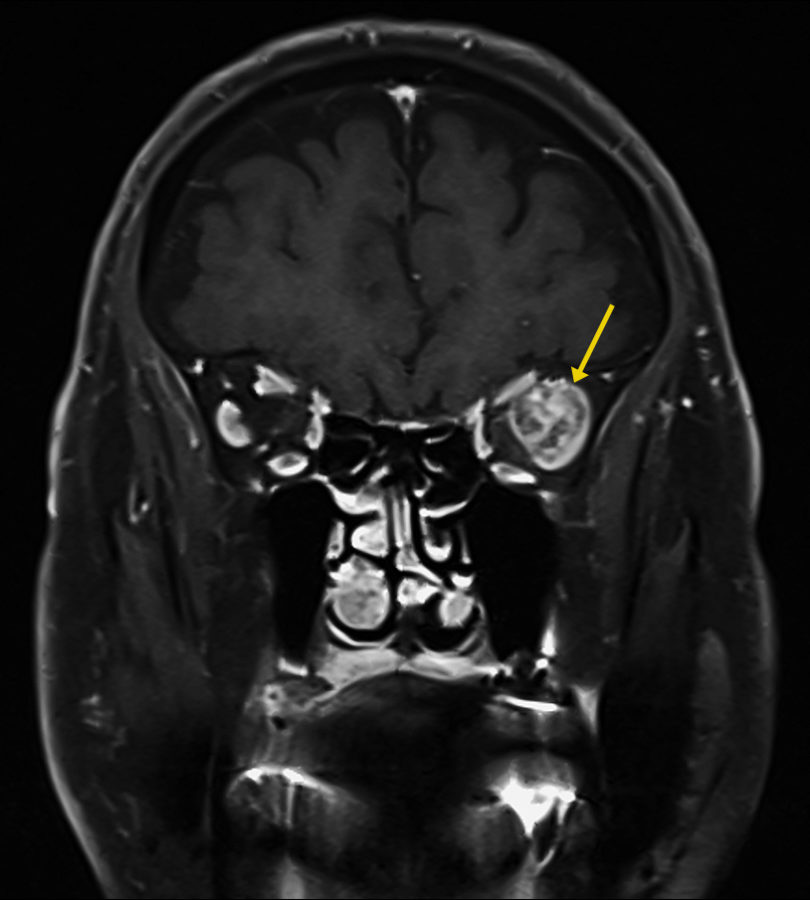
Figure 1: Coronal magnetic resonance T1-weighted imaging (T1-MRI) of the skull showing a roundish mass at the temporal aspect of the left orbit (arrow), with a highly heterogeneous intensity pattern, without apparent disruption of the orbital walls.
Case History
A 66-year-old Caucasian man presented with a complaint of tearing from his left eye (LE) for about 3 months. Ectoscopically, a slight elevation of the left upper lid crease was noticeable, without accompanying lid retraction (Figure 2, top). Uncorrected distance visual acuity (UDVA) was 10/10 bilaterally. Biomicroscopy was unremarkable, fundoscopy was indicative of mild hypertensive retinopathy, and intraocular pressure was within the normal range in both eyes. The LE had a discrete proptosis of 3mm (Figure 2, bottom), with no identifiable impairment of extraocular muscle function or blepharoptosis. There was neither a relative afferent pupillary defect nor a visual field defect. No mass could be detected on palpation. There was mild resistance to retropulsion of the globe, without pain, pulse or bruit.
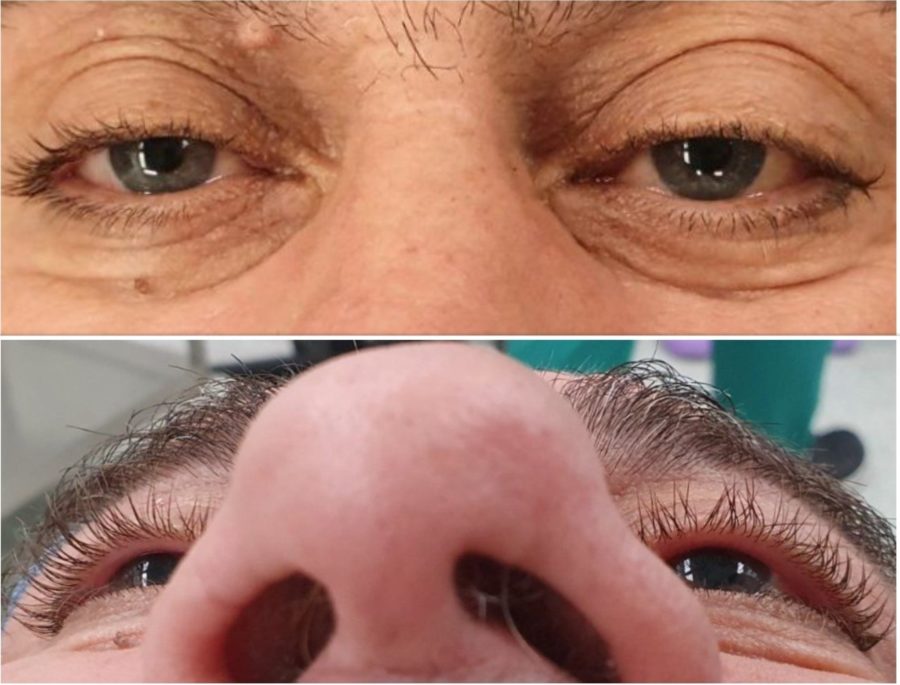
Figure 2: A slight elevation of the left upper lid crease is noticeable (top), with no lid retraction. A discrete proptosis is present on the left (bottom), without manifest ocular deviation.
Investigation proceeded with radiological imaging, rendering the detection of an irregular mass at the temporal aspect of the left orbit (Figure 1). Under magnetic resonance imaging, the tumor presented a fusiform disposition, following the lateral rectus muscle configuration (Figure 3). Following contrast injection, the mass displayed a characteristic enhancement pattern, with no clear delimitation from the muscle bundles.
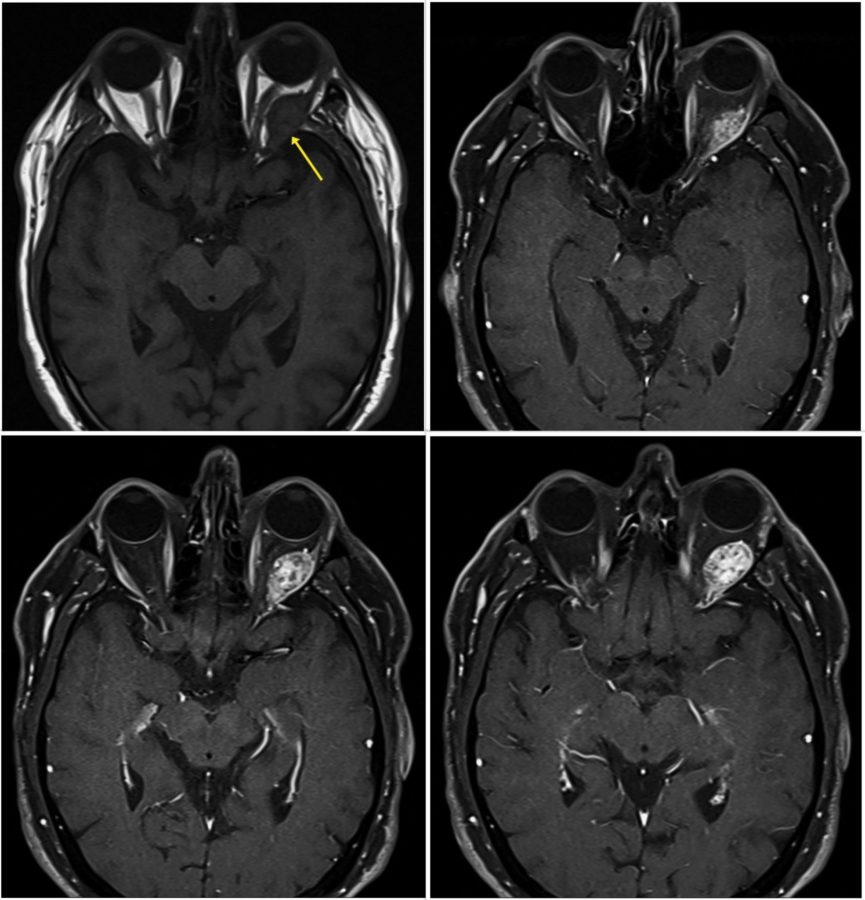
Figure 3: Axial MRI of the skull showing the high signal enhancement pattern of the orbital mass following contrast injection. Even though the tumor looks encapsulated at points (bottom right & left), other cuts suggest an intimate imbrication with the muscular tissue (top left).
A surgical approach was indicated and a frozen section biopsy was performed through a lateral orbitotomy. A reddish, highly vascularized mass was found in the belly of the lateral rectus muscle and extending to the apex of the orbital cone, covered by a pseudocapsule yet intricately mingled with the muscle fibers. In spite of the friable, non-encapsulated nature of the tumor, added to the technical difficulties imposed by its posterior location and tendency to bleed, an excisional debulking was carried out, without impairment of muscle contractility, damage to adjacent conal structures, or external disfiguration.
The surgical specimen was sent to an experienced pathologist for histological analysis. Microscopic examination of the neoplasm revealed bundles of large cells with clear or eosinophilic cytoplasm (Figure 4). The tumor cells exhibited strong positivity for the anti-cytokeratin monoclonal antibodies AE1 and AE3 (CKAE1/AE3) and the protein vimentin. The cells were also negative for CK7, and many showed nuclear positivity for paired-box gene 8 (PAX8). The histological morphology of the neoplasm, in combination with its immunophenotype, was consistent with the diagnosis of metastatic clear-cell renal carcinoma.
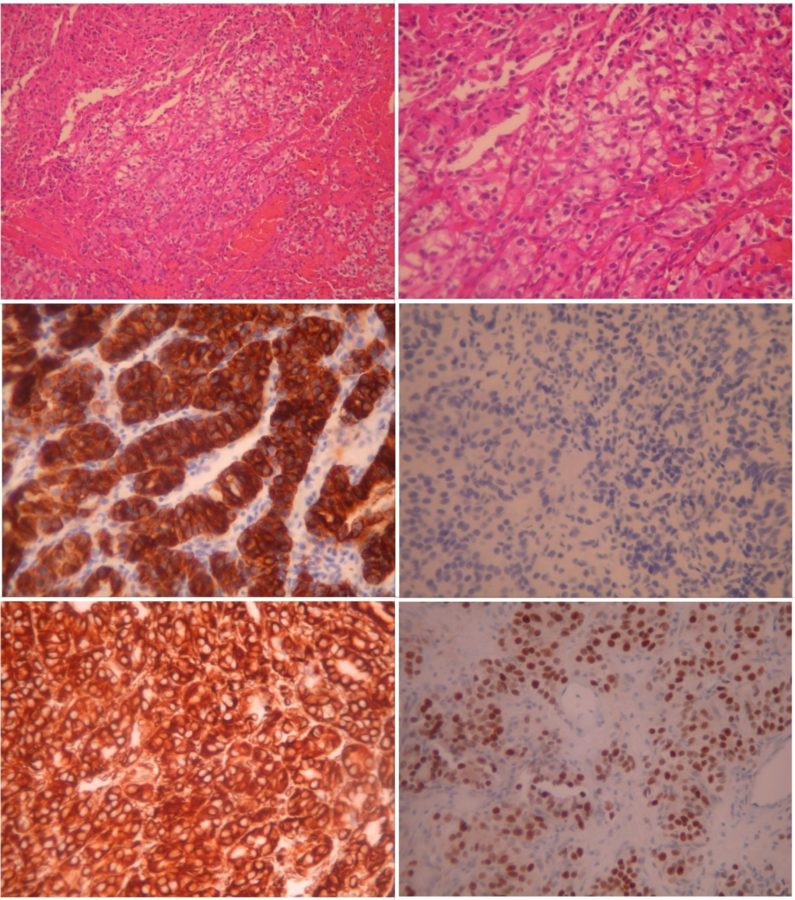
Figure 4: Microscopic photographs (histopathology) of the neoplasm, with the typical features of clear-cell renal carcinoma. Top left: hematoxylin-eosin stain (H+E) at 100X magnification (X 100) displaying large tumor cells with clear or eosinophilic cytoplasm. Top right: the same area in higher magnification (X 200). Middle left: Immunohistochemistry (IHC) X 200 showing the strong positivity of the tumor cells for CKAE1/AE3. Middle right: negative IHC for CK7 (X 200). Bottom left: strong positivity of the tumor cells for vimentin (IHC X 200). Bottom right: many tumor cells showing nuclear positivity for PAX8 (X 200).
Additional History
The patient had undergone a right nephrectomy due to renal carcinoma five years before. He also had a history of atrial fibrillation, an aortic aneurysm, and a surgical correction of inguinal hernia 6 months earlier. He was currently in use of sotalol, olmesartan, furosemide, rosuvastatin, and rivaroxaban (Xarelto®), this one being suspended before the orbital intervention.
Differential Diagnosis of Metastatic Orbital Carcinoma
- lymphoma
- hemangioma
- lymphangioma
- rhabdomyosarcoma
- schwannoma
- meningioma
It is nearly impossible to differentiate among orbital tumors based solely on history, physical and clinical exams. Radiological imaging can give clues as to the probable nature of the mass; however, the definitive diagnosis is obtained through biopsy.
Discussion and Literature
The improved survival of cancer patients, together with the longer aging of the population, has led to a higher incidence of patients living with metastatic disease in unusual sites such as the orbit and ocular adnexa. Besides, vigilant surveillance and advances in diagnostic techniques have increased the detection of orbital metastases (OM), even before disclosure of the primary site.
Metastatic orbital lesions account for 1 to 13% of all orbital tumors. Between 2 and 5% of patients with systemic cancer are reported to have an OM. Breast carcinoma accounts for most metastatic lesions of the orbit and ocular adnexa (48 to 53%). The other 2 most common primary cancers with OM are lung and prostate. Less usual sites include kidney, thyroid, stomach, skin (melanoma), and pancreas. Most OM are carcinomas. The most common symptoms are proptosis (52.3%) and relative afferent pupillary defect (38.7%) – due to direct compression of the optic nerve, usually at the orbital apex. Other clinical presentations include diplopia (48%), pain (19%), decreased vision (16%), ptosis (10%), and palpable mass.
The mean age of patients with OM is 60 years. The mean time of presentation after diagnosis of the primary cancer has been found to be 52 months; however, orbital metastasis can occur at any time after the initial cancer diagnosis. In more than 25% of cases, OM are the first manifestation of a primary tumor of unknown origin. In contrast to uveal metastases, OM are much more often unilateral than bilateral. Some metastases are very vascular (e.g., renal cell carcinoma) and biopsy can result in significant morbidity. Because the eye has no lymphatic channels, OM are secondary to hematogenous spread of the primary tumor. Therefore, the lungs are likely to be involved and must be investigated in these patients.
Considering an orbital mass in a patient with a cancer history, the anamnesis, physical examination, and imaging characteristics can help determine if the tumor must be suspected to be a metastasis. Due to the small space of the orbit and relatively rapid growth of most metastatic lesions, the presence of symptoms is suggestive of a metastatic orbital mass. Though any space-occupying mass – benign or malignant – may result in axial displacement of the globe, most metastases will produce a motility deficit, pain, or a decrease in vision. Benign lesions are usually asymptomatic, or symptoms may progress very slowly on the order of years. In the absence of previous cancer history, nonspecific signs involving other systems may guide the physician to a possible primary lesion; for example, hematuria may point to the kidneys as the probable site of origin.
Renal cell carcinomas (RCCs) are classified in several histological subtypes, clear-cell renal cell carcinoma (ccRCC) being by far the most common (70 to 75%). Clear-cell RCC usually affects patients over 50 years of age, and most cases (95%) are sporadic. Macroscopically, ccRCC is a solid, yellowish lesion with variable degrees of internal necrosis, hemorrhage, cystic degeneration and calcifications. Histologically, such lesions present clear cells because of their lipid- and glycogen-rich cytoplasmic content. Imaging findings are compatible with such histopathological features, denoting hypervascularized, heterogeneous lesions. Hematogenous metastases are relatively common in ccRCC, affecting mostly the lungs, bones, lymph nodes, and liver.
Carcinoma is the most common malignancy of the kidney, but only rarely does it metastasize to the orbit. Metastases to the head and neck have been found in 15% of RCC patients, most frequently involving the nose, paranasal sinuses, and oral cavity. Ocular metastases from kidney cancer usually involve the iris, ciliary body, and choroids, although eyelid and lacrimal sac involvement have also been described. Because these tumors can be confused with other amelanotic or vascular tumors, a high index of suspicion is required for early detection and management of the primary tumor, as this can affect the patient’s overall survival.
The clinical features of OM from kidney cancer are non-specific, therefore imaging is crucial to identify the ideal site for tissue biopsy or surgical approach. Imaging is also helpful in determining whether a mass can be a metastasis or not. Metastatic lesions are more common at the anterior rather than posterior orbit. Well-encapsulated, discrete, and focal intraconal masses are unlikely to be metastases, while masses involving the extraocular muscles or causing bone erosion are much more likely to be metastases. No area of the orbit is immune to metastasis, with cases having been reported to every anatomic structure of the orbit, including the subperiosteal space. Tumor patterns can vary from diffuse infiltrative to a focal mass. Metastases from renal cell carcinoma tend to be circumscribed and very vascular. As a rule, all OM should show some enhancement with contrast injection on MRI.
Orbital metastases often lead to some degree of functional impairment. A recent review of the literature showed that most OM have a preferential infiltration of soft tissues (40.2%), with 5.4% tumors extending intracranially. Histopathological evaluation is necessary to provide an accurate diagnosis, optimize clinical management, and estimate disease prognosis. An incisional biopsy can be obtained through a minimally invasive orbitotomy or, in selected cases, fine needle aspiration. Tumor debulking and excisional biopsy have the advantage of removing the mass, partially or as a whole, thus providing more adequate specimens for analysis and acting as therapeutic procedures. Symptom improvement is significantly higher after surgical resection when compared to biopsy only (p = 0.005).
The mortality rate for metastatic RCC has decreased significantly since 2012. Advances in diagnostic modalities and targeted therapies over the last 20 years have allowed significant improvement in the outcome for patients with metastatic RCC, including those with OM, leading to increased survival, better quality of life, and preservation of ocular function. Multidisciplinary management is the standard of care associated with the best results in these cases. Therapeutic options include surgical debulking or excision, hormonal therapy, chemotherapy, radiation therapy, and immunotherapy applying biological agents.
Keep in mind
- A rapid-onset, symptomatic, unilateral orbital mass should always raise the suspicion of a malignant lesion, possibly metastatic.
- Although rare, metastatic renal carcinoma must be included in the differential diagnosis of an orbital mass in a patient with a history of kidney cancer.
- Better survival rates and functional outcomes for metastatic orbital carcinoma depend on timely diagnosis and comprehensive treatment.
References
- Ahmad SM & Esmaeli B (2007). Metastatic tumors of the orbit and ocular adnexa. Current opinion in ophthalmology, 18(5), 405–413. https://doi.org/10.1097/ICU.0b013e3282c5077c
- Allen RC (2018). Orbital Metastases: When to Suspect? When to biopsy?. Middle East African journal of ophthalmology, 25(2), 60–64. https://doi.org/10.4103/meajo.MEAJO_93_18
- Palmisciano P, Ferini G, Ogasawara C, Wahood W, Bin Alamer O, Gupta AD, Scalia G, Larsen AMG, Yu K, Umana GE, Cohen-Gadol AA, El Ahmadieh TY & Haider AS (2021). Orbital Metastases: A Systematic Review of Clinical Characteristics, Management Strategies, and Treatment Outcomes. Cancers, 14(1), 94. https://doi.org/10.3390/cancers14010094
- Muglia VF & Prando A (2015). Renal cell carcinoma: histological classification and correlation with imaging findings. Radiologia brasileira, 48(3), 166–174. https://doi.org/10.1590/0100-3984.2013.1927
- Tan SY, Bastion MC & Mohd Khialdin S (2021). Orbital Tumor As First Manifestation of Metastatic Renal Cell Carcinoma. Cureus, 13(7), e16275. https://doi.org/10.7759/cureus.16275
- Vogele D, Sollmann N, Beck A, Haggenmüller B, Schmidt SA, Schmitz B, Kapapa T, Ozpeynirci Y, Beer M & Kloth C (2022). Orbital Tumors-Clinical, Radiologic and Histopathologic Correlation. Diagnostics (Basel, Switzerland), 12(10), 2376. https://doi.org/10.3390/diagnostics12102376
- Alkatan HM, Alyousef NA, Alshabib NS & Aljasser IHJ (2021). A comprehensive review of biopsy techniques for oculoplastic and orbital surgeons from ophthalmic pathologists’ perspective. Saudi Journal of Ophthalmology: Official Journal of the Saudi Ophthalmological Society. 2021 Jul-Sep;35(3):174-178. https://europepmc.org/article/med/35601859
- Mombaerts I, Ramberg I, Coupland SE & Heegaard S (2019). Diagnosis of orbital mass lesions: clinical, radiological, and pathological recommendations. Survey of ophthalmology, 64(6), 741–756. https://doi.org/10.1016/j.survophthal.2019.06.006
- Schick U, Lermen O & Hassler W (2006). Management of orbital metastases. Zentralblatt fur Neurochirurgie, 67(1), 1–7. https://doi.org/10.1055/s-2005-836922
- Coloma-González I, Ceriotto A, Amezquita-García E, Flores-Preciado J & Salcedo-Casillas G (2014). Orbital pulsatile metastasis as initial presenting sign of metastatic clear cell renal carcinoma. Archivos de la Sociedad Espanola de Oftalmologia, 89(12), 500–503. https://doi.org/10.1016/j.oftal.2013.11.012
- Magan T, Pradeep T, Tuluc M, Bilyk JR & Milman T (2022). Ocular adnexal metastases from renal cell carcinoma: An update and comprehensive literature review. Saudi journal of ophthalmology: official journal of the Saudi Ophthalmological Society, 35(3), 209–216. https://doi.org/10.4103/SJOPT.SJOPT_96_21
- Shome D, Honavar SG, Gupta P, Vemuganti GK & Reddy PV (2007). Metastasis to the eye and orbit from renal cell carcinoma–a report of three cases and review of literature. Survey of ophthalmology, 52(2), 213–223. https://doi.org/10.1016/j.survophthal.2006.12.004
- Mudiyanselage SY, Prabhakaran VC, Davis GJ & Selva D (2008). Metastatic renal cell carcinoma presenting as a circumscribed orbital mass. European journal of ophthalmology, 18(3), 483–485. https://doi.org/10.1177/112067210801800332
- Ho CC, Krishna KK, Praveen S, Goh EH, Lee BC & Zulkifli MZ (2010). Proptosis: a rare presentation of metastatic renal cell carcinoma. The Medical journal of Malaysia, 65(3), 229–230. http://www.e-mjm.org/2010/v65n3/Proptosis.pdf
- El-Hadad C, Koka K, Dong W, Do T, Haider M, Ursua JD, Ning J, Debnam JM & Esmaeli B (2021). Multidisciplinary Management of Orbital Metastasis and Survival Outcomes. Ophthalmic plastic and reconstructive surgery, 37(6), 541–545. https://doi.org/10.1097/IOP.0000000000001939


